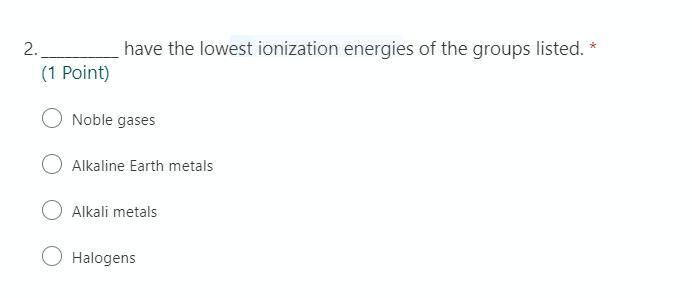Answers
Answer: have the lowest ionization energies of the groups listed
PLZ HELP I'LL AWARD BRAINLIEST
Explanation:
Related Questions
3.54 millilelvin = ? kelvin
Answers
Which is not the name of a family on the periodic table
a) Halogens
b) Noble Gases
c) Alkali Earth Metals
d) Actinides
Answers
;
Explanation: Look at the scrrn shot and you can see what is on there or not. :)))))
Actinides are not the name of the family of the periodic table.
Explanation:
In the modern periodic table :
Elements are arranged on the basis of their atomic numbersThere are 18 groups(vertical groups) and 7 periods (horizontal rows)Groups are also called families of the periodic table.Metals are present from the middle to the left-hand side of the periodic tableNonmetals are present on the upper right-hand side of the periodic tableMetalloids divide the periodic table in a zig-zag line with nonmetals on the right and metals on the left.The alkali metals are present under group 1The alkaline earth metals are present under group 2The noble gases are present under group 18The halogens are present under group 17The elements in separate two horizontal rows in the bottom are lanthanides and actinides.So, from this, we can conclude that actinides are not the name of the family of the periodic table.
Learn more about the periodic table:
brainly.com/question/226164?referrer=searchResults
brainly.com/question/1447213?referrer=searchResults
Li atoms in 3.7 moles
Answers
Answer:
We know that there are 6.022 * 10²³ atoms in 1 mole of any element
So, that means that there are 6.022 * 10²³ atoms in 1 mole of Li (Lithium)
Atoms in 3.7 Moles:
atoms in one mole = 6.022 * 10²³
atoms in 3.7 moles = 6.022 * 10²³ * 3.7
atoms in 3.7 moles = 22.28 * 10²³
atoms in 3.7 moles = 2.228 * 10²⁴
1. Consider the following: HCl (aq) + NaOH (aq) → NaCl (aq) + H2O (liq) ∆H = -57.62 kJ/mol
If a 25.0 mL of 0.144 M HCl (aq) at 25oC is added to 20.0 mL of 0.132 M NaOH (aq) at 25oC, calculate the final temperature of the contents. Assume the volumes are additive and that the resulting salt water solution has a density of 1.04 g/mL with a specific heat capacity of 3.93 J/goC. Note that you will also need to determine the limiting reactant.
Answers
Answer:
25.82°C
Explanation:
Based on the reaction, 1 mole of HCl and 1 mole of NaOH reacts, that means the reaction is 1:1. The moles of each compound are:
Moles HCl:
0.025L * (0.144mol/L) = 0.0036 moles HCl
Moles NaOH:
0.020L * (0.132mol/L) = 0.00264 moles NaOH
Thus, moles of reaction are 0.00264 moles
The heat released in a calorimeter is obtained using the equation:
Q = m*c*ΔT
Where Q is heat released in the reaction:
0.00264 moles * (-57.62kJ/mol) = 0.1521kJ = 152.1J of reaction
m is mass of the solution:
25.0mL + 20.0mL = 45mL * (1.04g/mL) = 46.8g
c is specific heat of the solution:
3.93J/gºC
And ΔT is change in temperature.
Solving for ΔT:
Q /mc = ΔT
151.2J / 46.8g*3.93J°C = 0.82°C = ΔT = Final temperature - Initial temperature.
Final temperature = 0.82°C + 25°C =
25.82°C
Rank these systems in order of decreasing entropy. Rank from highest to lowest entropy.
a. 1 mol carbon tetrafluoride gas at 273k 40L
b. 1 mol krypton gas at 273K 40L
c. 1/2 mol krypton gas at 100k 20L
d. 1 mol krypton gas at 273K 20L
e. 1/2 mol krypton liquid at 100K
f. 1 mol fluorine gas 273 K 40L
g. 1/2 mol krypton gas at 273K 20L
Answers
Answer:
The answer is "order will be A > F > B> D > G > C > E".
Explanation:
Entropy is disunity, the greater the lack of organization the entropy. When two gases use the same moles, then more entropy is achieved for a larger number of atoms in the molecule (even with more macrostates). Its smaller your volume the less the molecules may circulate, reducing as well as the amount of potential different countries and thus the entropy. Molecules or atoms in colder gas are much less active so they do not actually take so many various energy states and therefore less entropy. In colder gas. A liquid is requested more so than gas and its randomness decreases.In what ways are physical and chemical weathering alike? In what ways are they different?
Answers
When converting an “ordinary” number that is greater than 1 to scientific notation, how many non-zero digits are to the LEFT of the decimal point when you are finished?
Answers
Answer: 0 I think
Explanation:
pretty sure its zero because i learned it last year but im in middle school so you might want to look it up.
What is the predictable process in which one element type is converted into a new form?Immersive Reader
(10 Points)
A. Law of Superposition
B. Igneous formation
C. Fossil formation
D. Radioactive decay
Answers
Answer:
Radioactive decay
Explanation:
Answer:
D. Radioactive decay
Explanation:
If 25.6 mL isopropyl alcohol fully decomposes, what mass of H2 is formed? The density of isopropyl alcohol is 0.785 g/mL. g
Answers
Answer:
The correct answer is 0.67 g H₂
Explanation:
Isopropyl alcohol (C₃H₇OH) can decompose to give acetone (C₂H₆OH) and hydrogen gas (H₂) according to the following chemical equation:
C₃H₇OH (g) ⇒ C₂H₆CO(g) + H₂(g)
We can calculate the initial mass of isopropyl alcohol from the density and volume data:
density = m/V = 0.785 g/mL
⇒ m = density x V = 0.785 g/mL x 25.6 mL = 20.096 g C₃H₇OH
According to the chemical equation 1 mol of C₃H₇OH gives 1 mol H₂. The molar mass of C₃H₇OH is:
molar mass C₃H₇OH = (12 g/mol x 3) + (1 g/mol x 7) + 16 g/mol + 1 g/mol = 60 g/mol
molar mass H₂ = 1 g/mol x 2 = 2 g/mol
So, we obtain: 2 g H₂ from 60 g C₃H₇OH. We multiply this stoichiometric ratio (2 g H₂/60 g C₃H₇OH) by the initial mass of C₃H₇OH to obtain the mass of H₂ is formed:
20.096 g C₃H₇OH x (2 g H₂/60 g C₃H₇OH) = 0.6698 g ≅ 0.67 g H₂
Write both the complete electron-configuration notation and the noble-gas notation for a barium atom.
Answers
Answer:
Explanation:
Noble gas notation: [Xe] 6s2
Complete Electron Configuration: 1s22s22p63s23p63d104s24p64d105s25p66s2.
(ik kinda hard to understand but i looked it up ant it works)
Answer:
Complete Electronic Configuration:
1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁶5s²4d¹⁰5p⁶6s²
Electronic Configuration in Noble Gas Notation:
[Xe] 6s²
The process of reviewing information using an investigator is knowledge, training, experience, and expertise is what type of reasoning?
Answers
Answer:
Inductive reasoning
Explanation:
Inductive reasoning is based on making broad generalizations from specific observations. We draw conclusions from the data we have. This is the opposite of deductive reasoning, which starts with a general statement and aims to reach a specific conclusion.
Based on this, we can conclude that, if an investigator uses their knowledge, training, experience, and expertise to reach a conclusion, they are using inductive reasoning.
Which of these properties is physical property of aluminum
A. Aluminum rusts when it is exposed to moisture and air
B. Aluminum is a good conductor of heat
C. Aluminum reacts with oxygen to form aluminum oxide
D. Aluminum reacts with hydrochloric acid to give up aluminum chloride and hydrogen gas
Answers
Answer:
B. Aluminum is a good conductor of heat
Explanation:
Physical properties are usually those that can be observed using our senses such as color, luster, freezing point, boiling point, melting point, density, hardness and odor. The Physical Properties of Aluminum are as follows: Color : Silvery-white with a bluish tint.
Answer:
I know that it is D for sure
SOMEONE PLEASE HELPPP
Answers
True or False: Particles that are moving faster have a higher temperature
Answers
Answer:
true
Explanation:
I'm not sure why cause I dont know how to explain but it's TRUE
Answer:
True
Explanation:
The particles moving faster in a substance the hotter it gets.
In a fluorine atom, on which energy level are the valence electrons found?
Answers
Answer:
second energy level
Explanation:
Valence electrons are those electrons which are present in outer most orbital of the atom.
This can be easily found through the electronic configuration of atom.
Electronic configuration of F:
F₉ = 1s² 2s² 2p⁵
We can see that the valence electrons are present in second energy level of F atom.
There are seven valence electrons of fluorine.
It is called halogens.
Halogens are very reactive these elements can not be found free in nature. Their boiling points also increases down the group which changes their physical states. i.e fluorine is gas while iodine is solid.
Fluorine:
1. it is yellow in color.
2. it is flammable gas.
3. it is highly corrosive.
4. fluorine has pungent smell.
5. its reactions with all other elements are very vigorous except neon, oxygen, krypton and helium.
Consider the reaction of zinc metal with hydrochloric acid:
Zn(s) + 2 HCl(aq) → ZnCl2(aq) + H2(g).
If 29.39 × 1024 atoms of zinc completely reacted with hydrochloric acid, how many moles of hydrochloric acid must have reacted?
Do NOT include units in your entry. Report your answer with 3 SFs.
______________________ moles of HCl
Answers
Answer:
6.054×10²⁵
Explanation:
1)find number of moles of zinc
2)multiply the mole of zinc with 2
3)use the formula mol = number of particle/ avogadro constant
Total, 97.64 moles of hydrochloric acid (HCl) must have reacted.
To determine the number of moles of hydrochloric acid (HCl) that reacted, we first need to find the molar ratio between zinc (Zn) and hydrochloric acid (HCl) from the balanced chemical equation:
Zn(s) + 2 HCl(aq) → ZnCl₂(aq) + H₂(g)
From the equation, we see that 1 mole of Zn will reacts with 2 moles of HCl.
Given that 29.39 × 10²⁴ atoms of zinc reacted, we need to convert this quantity to moles. We can do this by using Avogadro's number:
1 mole of any substance = 6.022 × 10²³ atoms
Number of moles of zinc reacted = (29.39 × 10²⁴ atoms) / (6.022 × 10²³ atoms/mol)
Number of moles of zinc reacted ≈ 48.82 moles (rounded to 3 significant figures)
Now, using the molar ratio from the balanced equation, we can determine the number of moles of hydrochloric acid (HCl) that reacted:
Number of moles of HCl reacted = 2 × Number of moles of zinc reacted
Number of moles of HCl reacted ≈ 2 × 48.82 moles
≈ 97.64 moles
Therefore, approximately 97.64 moles of hydrochloric acid (HCl) must have reacted.
To know more about hydrochloric acid here
https://brainly.com/question/14519330
#SPJ2
I need help with this please
Thank you
Answers
Answer:
From fastest to slowest its: (4)A to B, (1)E to F, (3)C to D, (2)D to E
Explanation:
The steeper the line is the faster she went. D to E she didn't make any progress because the line is straight. Sry I'm terrible at explaining things.
determine the total amount of heat, in joules, required to completely vaporize a 50.0 gram sample of H20 at its boiling point at standard pressure
Answers
Answer: 1.13 x 10^5
Explanation:
The equilibrium constant for A + 2B → 3C is 2.1 * 10^-6
Determine the equilibrium constant for 2A + 4B → 6C.
a- 4.2 * 10^-6
b- 4.4 * 10^-12
c- 2.3 *10^11
d- 1.8 *10^-11
e- None of these
Answers
Answer:
b- 4.4 * 10^-12.
Explanation:
Hello.
In this case, as the reaction:
A + 2B → 3C
Has an equilibrium expression of:
[tex]K_1=\frac{[C]^3}{[A][B]^2}=2.1x10^{-6}[/tex]
If we analyze the reaction:
2A + 4B → 6C
Which is twice the initial one, the equilibrium expression is:
[tex]K_2=\frac{[C]^6}{[A]^2[B]^4}[/tex]
It means that the equilibrium constant of the second reaction is equal to the equilibrium constant of the first reaction powered to second power:
[tex]K_2=K_1^2[/tex]
Thus, the equilibrium constant of the second reaction turns out:
[tex]K_2=(2.1 * 10^{-6})^2\\\\K_2=4.4x10^{-12}[/tex]
Therefore, the answer is b- 4.4 * 10^-12.
Best regards.
experiment to determine the
ity of a free-falling object. Data
are presented in the table.
If the accepted value of acceleration due to gravity is
9.8 m/s2, how would you describe Clara's data?
Measurement
O precise but not accurate
accurate but not precise
both accurate and precise
neither accurate nor precise
9.3 m/s2
7.3 m/s2
7.5 m/s2
8.9 m/s2
Ok
Answers
Explanation :
Balance the equations by inserting coefficients as needed.
equation 1:
CaCO3 + HCl -> CaCl2 + CO2 + H2O
CaCO3+HCl⟶CaCl2+CO2+H2O
equation 2:
C6H12O2 + O2 -> CO2 + H2O
C6H12O2+O2⟶CO2+H2O
Answers
Answer:
1. CaCO3 + 2HCl → CaCl2 + H2O + CO2
2. C6H12O2 + 8O2 → 6CO2 + 6H2O
Explanation:
The balanced chemical equation is (i) CaCO3+2HCl⟶CaCl2+CO2+H2O
(ii) C6H12O2+ 8O2⟶CO2+6H2O
What is balanced chemical equation?An equation with equal amounts of every atom of an element on both endpoints of the arrow was called a balanced equation.
Given chemical equation is:
(i) CaCO3+HCl⟶CaCl2+CO2+H2O
It can be seen that in left side of the chemical equation count of chlorine atom is one while right side of the chemical equation it is two. So, by multiplying 2 as a coefficient in the right side of the equation. Balanced chemical equation will be
CaCO3+2HCl⟶CaCl2+CO2+H2O
(ii) C6H12O2+O2⟶CO2+H2O
It can be seen that, there are 12 hydrogen in the left side of the reaction while it is two hydrogen in the right side of the reaction. By multiplying 6 as a coefficient of hydrogen. Hence, the balanced chemical equation will be
C6H12O2+ 8O2⟶CO2+6H2O
The balanced chemical equation is
(i) CaCO3+2HCl⟶CaCl2+CO2+H2O
(ii) C6H12O2+ 8O2⟶CO2+6H2O
To know more about balanced chemical equation
https://brainly.com/question/15052184
#SPJ2
Pls help, and fast plsssssssss
Answers
Answer:
The cell on the left is animal, The cell on the right is a plant cell.
Explanation:
1: cell membrane
2: chloroplast
3: cell wall
4: vacuole
5: mitochondria
6:nucleus
7: lysosome
8: cytoplasm
Things to remember, only plant cells have cell walls. Plant cells are the only ones that have chloroplast.
2.
(3x – 4y = -10
(6x + 3y = –42
SOLUTION:
Answers
Answer:
Is like for solving for the solution for both equations??
Which element is classified as a noble gas?
Answers
Answer:
any of the seven chemical elements that make up Group 18 (VIIIa) of the periodic table. The elements are helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), radon (Rn), and oganesson (Og). :)
Explanation:
Answer:
For edge, the answer is;
D. Xe
(Xenon)
Explanation:
edg- answers ;p
Please help with number 7 thank you! 30 points
Answers
Answer:
X indicates proteins Y indicates lipids
Explanation:
X shows membrane bound proteins. Y shows lipids in the plasma membrane.
Answer:
I think that x indicates proteins and y indicates lipids
Explanation:
Proteins often are channels that help things get in and out of the cell, and x looks like one.
Membranes are made of phospholipids, which are types of lipids. Y is a lipid.
Can someone help me with this chemistry I have attached?, just helping me understand these problems. Its moles and questions
Answers
Kc = 3.07 x 10-4 at 24°C for 2NOBr(g) ↔ 2NO(g) + Br2(g). If the initial concentration of NOBr = 0.878 M, what is the equilibrium concentration (in M to 4 decimal places) of NO?
Answers
Answer:
The equilibrium concentration of NO is 0.02124 M.
Explanation:
Given that,
Initial concentration of NOBr = 0.878 M
[tex]k_{c}=3.07\times10^{-4}[/tex]
Temperature = 24°C
We know that,
The balance equation is
[tex]2NOBr\Rightarrow 2NO+Br_{2}[/tex]
Initial concentration is,
[tex]0.878\Rightarrow 0+0[/tex]
Concentration is,
[tex]-2x\Rightarrow 2x+x[/tex]
Equilibrium concentration
[tex]0.878-2x\Rightarrow 2x+x[/tex]
We need to calculate the value of x
Using formula of concentration
[tex]k_{c}=\dfrac{[NO][Br_{2}]}{[NOBr]^2}[/tex]
Put the value into the formula
[tex]3.07\times10^{-4}=\dfrac{[2x][x]}{[0.878-2x]^2}[/tex]
[tex]2x^2=3.07\times10^{-4}\times(0.878)^2+3.07\times10^{-4}\times4x^2-2\times2x\times0.878\times3\times10^{-4}[/tex]
[tex]2x^2=0.0002367+0.001228x^2-0.0010536x[/tex]
[tex]2x^2-0.001228x^2+0.0010536x-0.0002367=0[/tex]
[tex]1.998772x^2+0.0010536x-0.0002367=0[/tex]
[tex]x=0, 0.01062[/tex]
We need to calculate the equilibrium concentration of NO
Using formula of concentration of NO
[tex]concentration\ of\ NO=2x[/tex]
Put the value of x
[tex]concentration\ of\ NO=2\times0.01062[/tex]
[tex]concentration\ of\ NO=0.02124[/tex]
Hence, The equilibrium concentration of NO is 0.02124 M.
Which accurately represents these building blocks of matter from the smallest to the largest?
atom -- molecule or compound
O molecule -- atom - element
compound - molecule -- element
molecule atom or element
Answers
Answer:
A - Atom ---> molecule or compound.
Atomic radius is....
O The tendency for an atom to attract electrons
The energy required to remove an electron
O The energy required to add an electron
O The distance from the nucleus to the last orbital
Answers
Do you think there’s an advantage of process 1 over process 2 for this species?
Answers
Answer:
witch one witch species witch bone of 1 and 2
Answer:
Yes, process 1 has an advantage over process 2 for the planarian worm. Process 1 requires only one parent. If the planarian can’t find a mate, the life cycle will continue because it can still reproduce.
Explanation:
i did it