(d)
Aqueous potassium iodide reacts with aqueous copper(II) sulfate to produce iodine.
(i) Balance the chemical equation for this reaction.
Can someone please help
Answers
Explanation:
hope this helps you,
-s.
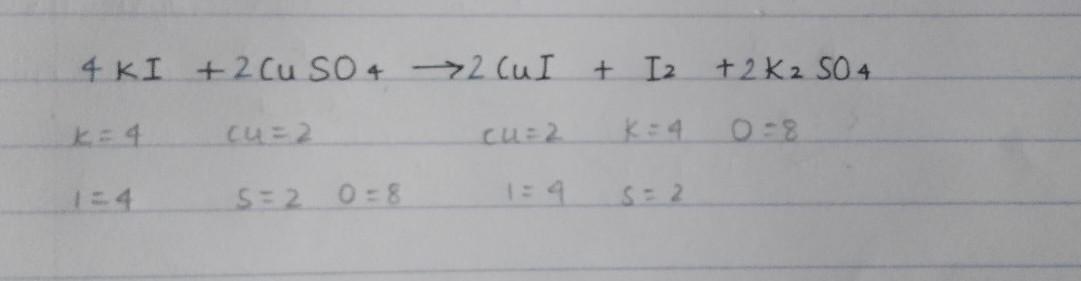
Aqueous potassium iodide reacts with aqueous copper(II) sulfate to produce iodine. The balance chemical equation for this reaction. 2CuSO4 + 4KI⟶ 2CuI + I2 + 2K2SO4.
What is balance chemical equation ?A chemical equation is said to be balanced if the quantity of each type of atom in the reaction is the same on both the reactant and product sides. In a balanced chemical equation, the mass and the change are both equal.
The chemical equation is said to be balanced if there are no inequalities. Every element in this illustration now has the same amount of atoms on the reactant and product sides.
The law of conservation of mass states that when a chemical reaction takes place, the mass of the reactants and products should be equal. As a result, during the chemical process, the number of atoms in each element remains constant. The chemical equation must be balanced as a result.
Thus, The balance chemical equation for this reaction. 2CuSO4 + 4KI⟶ 2CuI + I2 + 2K2SO4.
To learn more about balance chemical equation, follow the link;
https://brainly.com/question/28294176
#SPJ2
Related Questions
Explain why hydrogen, not sodium, is formed at the cathode.
Answers
Answer:
because of its electro affinity
Explanation:
it is electro positive
empirical or molecular? (C12H6)
empirical or molecular? (C2H4O)
empirical or molecular? (C8H8S4)
Answers
Answer:
C12H6 : Molecular
C2H4O : Empirical
C8H8S4 : Molecular
Explanation:
Molecular formula : tells you the exact number of atoms of each element are in a compound. If the number of elements can be simplified it is molecular.
Empirical formula : tells you the most reduced ratio of elements in a compound. If the number of elements is already in it's simplest form it's likely empirical.
C12H6 - this is molecular because C12H6 can further be simplified to C2H
C2H4O - this is empirical because C2H4O cannot further be simplified
C8H8S4 - this is molecular because C8H8S4 can further be simplified to C2H2S
A 6. 0 L container holds a sample of hydrogen gas at 150 kPa. The pressure increases to 2 atm and the temperature remains constant. What will the volume be? 0. 22 L 0. 44 L 2. 26 L 4. 50 L.
Answers
The volume of the container will be 0.45L.
What is Boyle's law?This law states that the product of volume and its pressure is constant for any gas.
Given, the Volume 1 of container 6.0L
V2 =?
Pressure 1 is 150 kPa
Increased pressure 2 is 2 atm
Temperature is constant.
By Boyle's law:
P1V1 = P2V2
[tex]150 \times 6 = 2\times V2 = 450[/tex]
Thus, V2 = 0.45 L.
Learn more about volume, here:
https://brainly.com/question/1578538
someone helppp pleaseee
Answers
Answer:
Top LineNebula → Average Star → Red Giant → Planetary Nebula → White Dwarf
Bottom LineNebula → Massive Star → Red Supergiant → Supernova → Neutron Star or Black Hole (for the second box)
-TheUnknownScientist 72
Answer:
top- nebula average star, reg giant, planetary nebula, white dwarf
bottom- nebula, massive star, red supergiant, supernova, neutron star-black dwarf
What are some of the difficulties in identifying particular drugs? Why is it important for forensic scientists to be able to identify particular drugs?
Answers
Answer:
The forensic scientist must be able to tell the difference between the substances.
Explanation:
It is important for forensic scientists to be able to identify particular drugs so they have evidence for the case that a certain drug was present.
The goal of forensic drug chemistry is to determine whether the material submitted contains an illegal substance.
How many moles of iron are present in 3.15 × 1024 atoms of iron?
Answers
Answer:
5.23 moles
Explanation:
Hope this helps!
What is the law of conservation of mass?
A. The total mass of materials present after a chemical reaction is the same as the total mass present before the reaction.
B. In a given compound, the relative numbers and kinds of atoms are constant
C. If two elements A and B combine to form more than one compound, the masses of B that can combine with a given mass of A are in the ratio of small whole numbers.
D. Each element is composed of extremely small particles called atoms.
Answers
Answer:
A. The total mass of materials present after a chemical reaction is the same as the total mass present before the reaction.
Explanation:
Law of conservation states that whatever mass you start with you will end with, mass cannot be gained or lost
A 5.00 kg weight is placed on a digital scale. The scale reads 4.98 kg. What
is the error of the scale measurement?
Answers
The error of this scale measurement is equal to 0.02 kg.
Given the following data:
Actual measurement = 5.00 kg.Measured value = 4.98 kg.To calculate the error of the scale measurement:
What is measurement?Measurement can be defined as an act (process) through which the weight, size, magnitude or distance traveled by an object or body is taken, especially for the purpose of an experiment.
Mathematically, the error of a scale measurement is given by this formula:
[tex]Error = Actual \;measurement-Measured \;value[/tex]
Substituting the given parameters into the formula, we have;
[tex]Error = 5.00-4.98[/tex]
Error = 0.02 kg.
Read more on measurement here: https://brainly.com/question/26374907
What is the percentage of oxygen in carbon dioxide?.
Answers
Answer:
72.7%
Explanation:
The first thing you need to do here is to figure out the mass of oxygen in
1 mole of carbon dioxide. To do that, you must use the compound's molar mass.
Now, carbon dioxide has a molar mass of 44.01 g mol−1. This means that 1 mole of carbon dioxide has a mass of 44.01 g. Oxygen has a molar mass of 16.0 g mol−1, so 1 mole of oxygen atoms has a mass of 16.0 g.
This means that if you take 44.01 g of carbon dioxide, you know for a fact that it will contain: 32g oxygen.
This means that 100 g of carbon dioxide will contain:
100gCO2 × 32gO2 / 44.01gCO2 = 72.7 g Oxygen
Therefore, carbon dioxide has a percent composition of 72.7% oxygen, i.e. for every 100 gof carbon dioxide you get 72.7 g of oxygen.
Number of energy levels containing electrons in iodine.
Answers
Answer:
First Energy Level: 2
Second Energy Level: 8
Third Energy Level: 18
Fourth Energy Level: 18
Element: Iodine
Group: 17
No. of electrons/shell: 2, 8, 18, 18, 7
What happens when a neutral object is brought near a positive object?
Answers
Answer:
stick together like magnets
Explanation:
Answer:
If a positively charged body is brought near to a neutral or uncharged body, it induces a negative charge on the near side and a positive charge on the far side of the neutral object. This creates a force of attraction between the two bodies.
What are comets made of?
A. Dust, sand, and hydrogen
B. Rock, metal, and liquid water
C. Dust, metal, and helium
D. Rock, dust, and ice
SUBMIT
Answers
Answer:
C. Rock, dust, and ice.
Explanation:
Comets are frozen leftovers from the formation of the solar system composed of dust, rock, and ices.
If 4.00 moles of fluorine are reacted with excess HBr, how many grams of bromine will be formed
Answers
The mass of bromine that would be formed is 639 g
StoichiometryFrom the question,
We are to determine how many grams of bromine would be formed
First, we will write the balanced chemical equation for the reaction,
The balanced chemical equation for the reaction is
F₂ + 2HBr → 2HF + Br₂
This means, 1 mole of fluorine reacts with 2 moles of HBr to produce 1 mole of bromine.
Then,
4.00 moles of fluorine will react with excess HBr to produce 4.00 moles of bromine.
Therefore,
The number of moles of bromine produced is 4.00 moles
Now, for the mass of bromine formed,
Using the formula,
Mass = Number of moles × Molar mass
Molar mass of bromine = 159.808 g/mol
Then,
Mass of bromine formed = 4.00 × 159.808
Mass of bromine formed = 639.232 g
Mass of bromine formed ≅ 639 g
Hence, the mass of bromine that would be formed is 639 g.
Learn more on Stoichiometry here: https://brainly.com/question/24691353
Question 10(Multiple Choice Worth 5 points)
(04.05 LC)
During which of the following processes would carbon most likely be required?
O Breaking of rocks
O Generation of electricity
O Making of glucose
O Running of cars
I’m lost
Answers
Answer:
O Generation of electricity
Explanation:
because the others dont make sense to me and tbh carbon is used everyway and everywhere
When you measure the temperature of hot soup you're measuring the
Answers
Answer:
Amount of Thermal Energy
Explanation:
How many moles is 2.80 × 1024 atoms of silicon? A. 4.65 mol B. 0.465 mol C. 2.15 mol D. 6.02 mol
Answers
Answer:
A) 4.65mol
Explanation:
[tex]\frac{2.80x10^{24}atoms }{1} *\frac{1 mol}{6.02x10^{23}atoms } =4.65mol[/tex]
List one symbol for an element that forms an oxidation number of
-2?
Answers
Answer:
Sulfur
Explanation:
S+2e−→S2−
Which fatty acids are considered essential fatty acids?.
Answers
Answer:
alpha-linolenic acid and linoleic acid
Explanation:
Hope this helps!
number of protons in nucleus of magnesium atom????
Answers
Pure maple syrup has a high sugar content. Its composition is 70% sucrose (C12H2011). If you heat maple
syrup what will happen to its viscosity?
answer : It will decrease
Answers
Answer:
it will decrease
Explanation:
Answer:
A. it will decrease
Explanation:
trust
Imagine that the sun disappeared from the universe tomorrow. Which sphere or spheres would this affect?(1 point)
Answers
The earth and other planets start drifting into space.
What happen if sun disappear?If the sun disappear from the universe, then Earth would be drawn to a new center of gravity. The gravity of Earth and the other planets of the solar system adversely affected due to no constant supply of energy from the sun which leads to drifting of earth into space.
In conclusion, the earth and other planets start drifting into space.
Learn more about universe here: https://brainly.com/question/805395
50 points
PLEASE HELp this dropped most of grade but I don’t understand at all
Answers
Answer:
that number formula units of mole that meansis You need to learn it on Yt
Example:
There are 6.022×1023 in 1 mole of anything, including formula units. You need to determine the number of moles in 0.335 g CaO . Once you know the number of moles of CaO , you can determine the number of formula units by multiplying the number of moles by 6.022×1023
Why do you not fill you car tires with just oxygen?
Answers
Answer:
in text
Explanation:
But the main reason for using pure nitrogen is resistance to leakage. Nitrogen molecules find it harder to sneak out through the tire past the rubber molecules than oxygen molecules. This makes nitrogen a good bet for race car, aircraft and heavy-duty equipment where precise or constant pressure is critical.
BRAINLIEST PLEASE !!!!
PLease help my imma mark brainlist plsss
Did the chemical reaction absorb or release energy? How do you know?
Answers
Answer:
Chemical reactions that absorb (or use) energy overall are called endothermic. In endothermic reactions, more energy is absorbed when the bonds in the reactants are broken than is released when new bonds are formed in the products.
what is the area of the face of a rectangle crystals that has a widlth (w) measuring 3.4 cm and a length (1) measuring 4.2 cm?
Answers
Answer:
14.28 [tex]cm^{2}[/tex]
Explanation:
Multiply length by width to get area: 3.4 x 4.2 = 14.28
The answer will be in units of cm x cm = [tex]cm^{2}[/tex]
A chemist carefully measures the amount of heat needed to raise the temperature of a sample of from to. The experiment shows that of heat are needed. What can the chemist report for the molar heat capacity of ? round your answer to significant digits.
Answers
Answer:
Are there any options or starting points for this question?
Explanation:
Which of the following is made of an element?
Rust
A rose
diamond
Blue paint
O None of these
Answers
A buffered solution has a pH of 7.5. What would happen to the pH if a small
amount of acid were added?
A. The pH would stay at about 7.5.
B. The pH would become less than 7.0.
C. The pH would increase to greater than 7.5.
D. The pH would decrease to 7.0.
Answers
For this buffered solution with a pH of 7.5, B. The pH would become less than 7.0.
What is pH?In Science, pH literally means the power of hydrogen ions and it can be defined as a measure of the molar concentration of hydrogen ions in a particular solution. Thus, it is typically used to specify the acidity, neutrality or basicity of any chemical solution.
On a pH scale, a solution with a pH of 7 is neutral while a solution with a pH below 7 is acidic and it's basic (alkaline) when its pH is above 7.
In this scenario, a buffered solution with a pH of 7.5 would become less than 7.0 when a small amount of acid is added to it.
Read more on power of hydrogen here: https://brainly.com/question/24242819
Which equation below is an example of a single-replacement reaction?
A.6CO2(g) + 6H2O(l) → C6H12O6(aq) + 6O2(g)
B.H2SO4(aq) + 2NaOH(aq) → Na2SO4(aq) + 2H2O(l)
C.Ca(OH)2(s) Δ→ CaO(s)+ H2O(l)
D.Zn(s) + 2HCl(aq)→ ZnCl2(aq) + H2(g)
Answers
The equation that depicts a single replacement reaction would be: Zn(s) + 2HCl(aq)→ ZnCl2(aq) + H2(g)
Single replacement reactionsThey are also known as single displacement reactions.
They are reactions in which one element takes the place of another in a compound. That is, one element replaces another in a compound.
Looking at all the reactions from A - D, one can see that the only reaction that exemplifies a single replacement reaction is D.
Here, Zn replaced H in HCl.
More on single replacement reactions can be found here: https://brainly.com/question/13328989
Which substances have Delta. Hf = 0 kJ/mol by definition? Select all that apply. O2(g) N(g) H2O(l) Br2(l) Fe(s) He(g).
Answers
The substances that have Delta Hf = 0 kJ/mol are O2(g), Br2(l), He(g), Fe(s).
What is delta Hf = 0?Delta Hf = 0 is the standard enthalpy of any element in its most stable form is equal to zero.
When enthalpy is negative, the delta Hf is minus zero, which means the system releases heat.
When the system gains heat, the delta Hf is positive.
Thus, the substances are O2(g), Br2(l), He(g), Fe(s).
Learn more about Delta Hf, here:
https://brainly.com/question/25912291
Answer:
1,4,5,6
Explanation: