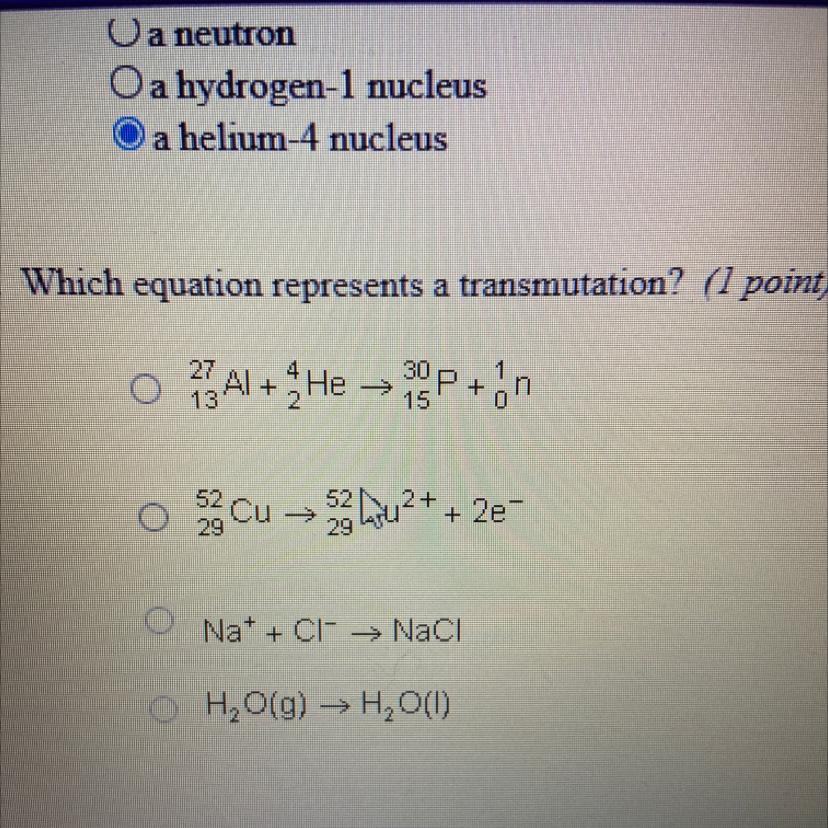
Answers
Answer:
A
Explanation:
edge21
Related Questions
which is radioactive decay
Answers
Answer:
Explanation:all
What is a empirical formula
Answers
Answer:
The empirical formula of a compound is the simplest whole number ratio of atoms of each element in the compound. It is determined using data from experiments and therefore empirical.
For example, the molecular formula of glucose is C 6H 12O 6 but the empirical formula is CH 2O.
Please mark as brainliest if answer is right
Have a great day, be safe and healthy
Thank u
XD
Answer:
a chemical formula showing the simplest ratio of elements in a compound rather than the total number of atoms in the molecule CH2O is the empirical formula for glucose.
what mass of oxygen reacted with the 8.40 grams of c
Answers
differences between geometric isomerism and optical isomerism?
Answers
Geometric isomers have the same structural formulas but differ in the arrangement of groups at a single atom, at double bonds, or in rings. ... One of the optical isomers rotates the light in one direction, the other rotates the light in the opposite direction but by the same amount.
Why doesnt the gas on a gas giant escape into space, as it has on mercury?
Answers
Answer:
When surface gravity is ore than the escape velocity gases can not escape from the planet.
Explanation:
Mercury is near Sun and the solar wind is also blowing out the atmosphere.
The gas on a gas giant escape into space, as it does on mercury because mercury is closest to sun.
What is a gas giant?A gas giant is defined as a giant planet which is composed mainly of elements of hydrogen and helium. Gas giants are also called failed stars because they contain the same basic elements as a star. Jupiter and Saturn are the gas giants of the Solar System. The term "gas giant" was originally synonymous with "giant planet". However, in the 1990s, it became known that Uranus and Neptune are really a distinct class of giant planets, being composed mainly of heavier volatile substances.For this reason, Uranus and Neptune are now often classified in the separate category of ice giants.
Jupiter and Saturn consist mostly of elements of hydrogen and helium, with heavier elements making up between 3 and 13 percent of their mass. They are thought to consist of an outer layer of compressed molecular hydrogen surrounding a layer of liquid metallic hydrogen, with probably a molten rocky core inside.
Learn more about gas giant,here:
https://brainly.com/question/9401838
#SPJ2
How many sigma and pie bonds are in the following compound?
1.00
on
H:0:
| ||
H-C-C-H
1
H
Answers
For the first
H : O : H
Theres two bonds here
Each other are sigma
So 2Sigma bonds present
For the Second
I believe you wanted to write Ethyne....
In a triple bond... Theres 1sigma and 2 Pi bonds
Every single bond is a sigma bond
So
Counting all
we have
2Pi bonds and 3Sigma bonds
A molecule with 14 total electrons and 12 total protons
Answers
Answer:
Explanation:
Tenochtitlan was located on a swampy island in Lake Texcoco in what is today south central Mexico. The Aztecs were able to settle there because no one else wanted the land. At first, it wasn't a great place to start a city, but soon the Aztecs built up islands where they could grow crops. The water also worked as a natural defense against attacks from other cities.
Read more at: https://www.ducksters.com/history/aztec_empire/tenochtitlan.php
This text is Copyright © Ducksters. Do not use without permission.
The charge on a molecule with 14 electrons and 12 protons = -2
Although your question is vague a general answer is provided above
When the number of protons and the number of electrons in a molecule are equal to each other, the charge on the molecule will be neutral, this is because Electrons are negatively charged while protons are positively charged.
Therefore a molecule with 14 electrons and 12 protons will have a charge of
= -14 + 12 = - 2
learn more : https://brainly.com/question/18169295
Can anyone please help?
Answers
Answer:
Earth
Explanation:
Earth is unique in the fact that we have an oxygen-rich atmosphere
by what process does water vapor become a cloud?
a. evaporation
b. transpiration
c. condensation
d. precipitation
Answers
At a temperature of 408K, which gad will have the highest velocity?
Answers
Answer:
1 - NO2 at 339 K
2 - Ne at 371 K
3 - H2 at 371 K
4 - H2 at 425 K
Explanation:
Kinetic Energy is directly related to temperature; the higher the temperature the higher the kinetic energy. Kinetic energy is also equal to 12m⋅v2, so if we want a high velocity we want high temp and low mass. So let's list out approximate masses:
m(H2)≈2
m(NO2)≈46
m(Ne)≈20
So we have NO2 at 339 K, the lowest temperature out of the mix, and the highest mass out of the mix, so this is moving the slowest.
In contrast, we have H2 at 425 K, the highest temperature out of the mix, and the lowest mass out of the mix, so this is moving the fastest.
Now we have Ne and H2 at 371 K, since they are at the same temperature they have the same kinetic energy. But H2 is lighter than Ne so it must be faster. To quantify this mathematically, let's assume (this is wrong but just as an assumption for an example) KE at 371 K is 100:
100=12⋅m⋅v2
200=m⋅v2
√200m=v
So H2 is about v=10 and Ne is about v=√10≈3
So the order to recap is:
1 - NO2 at 339 K
2 - Ne at 371 K
3 - H2 at 371 K
4 - H2 at 425 K
Hope that makes it clearer!
For many purposes we can treat butane as an ideal gas at temperatures above its boiling point of . Suppose the pressure on a sample of butane gas at is cut in half. Is it possible to change the temperature of the butane at the same time such that the volume of the gas doesn't change? yes no If you answered yes, calculate the new temperature of the gas. Round your answer to the nearest °C.
Answers
Answer:
A. Yes
B. The new temperature of the gas is -116 °C
Note: The question is incomplete. The complete question is given below :
For many purposes we can treat butane C H10) as an ideal gas at temperatures above its boiling point of - 1. °C. Suppose the pressure on a 500 mL sample of butane gas at 41.0°C is cut in half. Iyes Is it possible to change the temperature of the butane at the same time such that the volume of the gas doesn't change? yes no If you answered yes, calculate the new temperature of the gas. Round your answer to the nearest °C.
Explanation:
A. According to the pressure law of gases,for a fixed mass of gas the pressure of a gas is directly proportional to its Kelvin temperature once the volume is kept constant. This means that a change in temperature can bring about a change in pressurein a gas at constant volume.
B. From the pressure law of gasese: P1/T1 = P2/T2
Where initial pressure = P1, final pressure = P2
Initial temperature = T1, final temperature = T2
For the butane gas;
P1 = P
P2 = P/2
T1 = 41°C = (273 + 41 ) K = 314 K
T2 = ?
From the equation, T2 = T1 × P2 / P1
T2 = 314 × P/2 /P
T2 = 157 K
T2 = (157 - 273) °C = -116 °C
Therefore, the new temperature of the gas is -116 °C
describe and compare the trends in hardness for group 1 and group 2
elements?
Answers
Answer:
The key difference between group 1 and group 2 elements is that all group 1 elements have unpaired electrons in their outermost orbital, whereas group 2 elements have paired electrons in their outermost orbital.
The hardness of group 2 elements are harder than group 1 elements because they have a strong metallic bond and atoms are closely packed.
What are the group 1 and 2 elements?The group 1 elements are alkali metals and group 2 elements are alkaline earth metals. Group 1 contain 7 elements. Group 2 has 6 elements.
The difference between, group 1 elements have unpaired electrons in their outermost orbital, whereas group 2 elements have paired electrons in their outermost orbital.
Thus, the hardness of group 2 elements are harder than group 1 elements because they have a strong metallic bond and atoms are closely packed.
Learn more about group 1 and 2 elements
https://brainly.com/question/867686
#SPJ2
In a receptacle we have 29 g of hydrochloric acid that react with an excess of ammonia according the following equation:
HCl + NH3 → NH4Cl
The percent yield of the reaction is 54 %. Determine:
1.Mass of ammonium chloride obtained.
2.How many moles of ammonium chloride are forme
Answers
Answer:
2969429th Ave
Explanation:
i dont know
Calculate the kinetic energy of a mole of oxygen gas molecules that have a speed
of 10.0 m/s.
Answers
Answer:
1600J
Explanation:
1 mole of oxygen gas (O2) has a mass of 32g i.e. the molar mass = 32g/mol
Kinetic energy (K.E) = ½ × m × v²
Where;
m = mass (g)
v = speed or velocity (m/s)
From the information given in this question;
m = 32g
V = 10m/s²
K.E = ½ × 32 × 10²
K.E = 16 × 100
K.E = 1600J
What is the percent yield if the actual yield of
NH3 is 70 grams
Answers
1. What is the difference between a synthetic resource and a Natural Resource? This is technology.and I need help pls help!!!!!!!!!!!!!
Answers
Answer:
Natural materials are those that are found in nature and have not been made by humans. By comparison, synthetic materials are man-made and cannot be found in nature. Synthetic products are usually created in laboratories by mixing different chemicals, or prepared compounds and substances made in a laboratory.
Explanation:
Natural materials are those that are found in nature and have not been made by humans. By comparison, synthetic materials are man-made and cannot be found in nature. Synthetic products are usually created in laboratories by mixing different chemicals, or prepared compounds and substances made in a laboratory
A tau lepton decays into an electron, an electron antineutrino and a tau neutrino. Write out this reaction in symbolic (equation) form and show that charge and lepton number is conserved. 2. What is the total number of quarks in a helium nucleus consisting of 2 protons and 2 neutrons
Answers
The equation in the symbolic form can be written as,
[tex]\tau \;- > e- + {\bar{\nu }} e + \nu\tau[/tex]
The total number of quarks in a helium nucleus consisting of 2 protons and 2 neutrons is 12 quarks.
What is the meaning of lepton?Any of a family of particles (such as electrons, muons, and neutrinos) that have spin quantum number ¹/₂ and that experience no strong forces.
The equation in the symbolic form can be written as,
[tex]\tau \;- > e- + {\bar{\nu }} e + \nu\tau[/tex]
Charge on the left side = -1
Charge on the right side = -1+0+0 = -1
As the charge on the electron antineutrino, [tex]{\bar{\nu }}[/tex] e and the tau neutrino, [tex]\nu\tau[/tex] are zero each.
Thus, charge on left side = charge on right side
Or, the equation is in accordance with charge conservation.
Lapton number of left side = 1
Laptop number of right side = 1-1+1 = 1
This is because, lapton number of the particles involved are,
Tau lapton, [tex]\tau[/tex]-1 = 1,
Electron, e-1 = 1,
Electron antineutrino, [tex]{\bar{\nu }}[/tex]e = -1,
Tau neutrino, [tex]\nu\tau[/tex]= 1,
Thus, lapton number of left side = lapton number of right side
So, the equation obeys lapton number conservation.
2.
The total number quarks in a helium nucleus which is composed of 2 protons (positively charged particle) and 2 neutrons (uncharged particle) is:
Since there 3 quarks in each nucleon, 4 nucleons would have a total of 12 quarks.
Just simply multiply 3 quarks by the number of nucleons:
So, 3 (quarks) x 4 (nucleons) = 12 quarks
Therefore, there are 12 quarks in 4 nucleons.
Learn more about lepton decays here:
https://brainly.com/question/12044097
#SPJ1
What major component of water affects most its properties?
A. its abundance on Earth
B. its different states
C. polarity
D. its expansion when freezing
Answers
Answer:
C. polarity
Explanation:
Water is polar that is why it is a universal solvent.
A 5.0 L sample of gas at 300. K is heated to 600. K. What will the new volume of the gas be?
Answers
Answer:
[tex]V_2=10L[/tex]
Explanation:
Hello there!
In this case, according to the given information, it turns out possible for us to calculate the required new volume by using the Charles' law as a directly proportional relationship between temperature and volume:
[tex]\frac{V_2}{T_2} =\frac{V_1}{T_1}[/tex]
In such a way, we solve for V2 and plug in V1, T1 and T2 to obtain:
[tex]V_2=\frac{V_1T_2}{T_1}\\\\V_2=\frac{5.0L*600K}{300K}\\\\V_2=10L[/tex]
Regards!
Commercial soaps are mixtures of ionic compounds typically made up of monatomic cations, such as Na and K , and organic polyatomic anions derived from fatty acids. These negatively charged molecular ions are characterized by the presence of hydrocarbon chains which are 12 to 18 carbon atoms long. How hard (solid, insoluble) or soft (liquid, soluble) a soap is depends on the nature of the anions and cations present in the system. Analyze how each of the following factors may affect the hardness or softness of soaps:
1. The nature of the cations. For example, Na* vs Li* vs K.
2. The length of the hydrocarbon chain. For example, 12 carbons (laureate lon), 14 carbons (myristate lon), or 18 carbons (stearate lon).
Answers
Answer:
Following are the solution to the given question:
Explanation:
For question 1:
The sodium soap containing Na+ is strong whereas the softer or liquids were potassium soap.It's hard to use lithium soap.These Na+, K+, and Li+ ions act as the hydrophilic center.Calcium and Magnesium ions could be substituted by hard water with increasing hydrophilicity.For question 2:
The hydrophobicity of its carbon chain increases but one appears weaker with only an increased length.Therefore, the laureate is hard, while the stearate is soft.The oceanic crust is destroyed at convergent boundaries because it_______________________ *
a) diverges to form a rift valley
b) goes into a subduction zone where it is melted by the hot magma
c) transforms
d) the rock crumbles at an ocean ridges
Answers
Answer: I believe the answer is d) the rock crumbles at an ocean ridges
Explanation:
HELLP ME PLSSS
The passing of heat through a material is called ________.
A. vibration
B. conduction
C. radiation
D. convectio
Answers
Answer:
B
Explanation:
conduction is the transfer of heat between objects that touch
PLEASE HELP EMERGENCY ‼️‼️ needed ASAP
Answers
Answer:
the photo is kind of blurry i cant really see it, sorry
Explanation:
Which base(s) is weaker than ammonia?
hydroxylamine, methylamine, and pyridine
pyridine only
hydroxylamine and methylamine
hydroxylamine and pyridine
Answers
Answer:
Hydroxylamine and pyridine
Explanation:
Just did it
Calculate the ionization constant for the following acids or bases from the ionization constant of its conjugate base or conjugate acid: Keep your answer to 2 significant figures (CH3)3NH+
Answers
Answer:
7.41 × 10⁻⁵
Explanation:
Let's consider the basic dissociation reaction of trimethylamine (CH₃)N).
(CH₃)N + H₂O = (CH₃)NH⁺ + OH⁻
According to Brönsted-Lowry, in this reaction (CH₃)N is a base and (CH₃)NH⁺ is its conjugate acid. The pKb for (CH₃)N is 9.87. We can calculate the pKa of (CH₃)NH⁺ using the following expression.
pKa + pKb = 14
pKa = 14 - pKb = 14 - 9.87 = 4.13
Then, we can calculate the acid dissociation constant for (CH₃)NH⁺ using the following expression.
pKa = -log Ka
Ka = antilog - pKa = antilog -4.13 = 7.41 × 10⁻⁵
Calculate the Ka of a 0.35M weak acid with a pH of 4.2.
Answers
Answer:
Ka = 1.14x10⁻⁸
Explanation:
First we calculate [H⁺] from the pH:
pH = -log[H⁺][H⁺] = [tex]10^{-pH}[/tex][H⁺] = 6.31x10⁻⁵ MFor a monoprotic weak acid, the molar concentration of H⁺ of a solution can be expressed as:
[H⁺] = √(C*Ka)Where C is the molar concentration of the weak acid solution.
6.31x10⁻⁵ M = [tex]\sqrt{0.35M*Ka}[/tex]1.14x10⁻⁸ = Kapls help me with is question.
Answers
Answer:
25cm³ = 0.025L of H2So4
Molarity of H2SO4 = 1moldm-³
Recall ... 1dm-³ = 1L
So the Molarity can also be 1mol/L
Mole = Molarity x volume in L
Mole of H2SO4 = 1mol/L x 0.025L
=0.025Moles of Sulphuric acid reacted.
From the equation of reaction
1mole of H2SO4 reacts to produce 1mole of Copper sulphate crystal
Since their Mole ratio is 1:1
It means that Since 0.025mole of H2SO4 reacted.... 0.025mole of CuSO4.5H2O would be produced
Nice.. OK
So we know the moles of CuSO4.5H2O produced
We can get the Mass
Recall
From
Mole=Mass/Molar Mass
Mass = Mole x Molar Mass
Molar Mass of CuSO4.5H2O = 64 + 32 + 16x4+ 5(2+16)
Mm= 250g/mol
Mass = 0.025mol x 250g/mol
= 6.25g of CuSO4.5H2O crystals Would be PRODUCED.
Uranium is an element with three naturally occurring isotopes: 238U, 235U, and 234U. This means that 238U, which has a mass number of 238 has _______more than 235 u which has a mass number of 235.
Answers
Answer:
The correct answer is - neutrons.
Explanation:
Uranium has various isotopes found naturally that are three 238U, 235U, and 234U. Uranium has an atomic number of 92 which means there are 92 protons and 92 electrons in the atomic structure.
Isotopes have the same number of protons but a different number of neutrons that can vary from 141 to 146. U-238 has 146 neutrons in the nucleus, whereas 235 U has 143 neutrons.
The U- 238 has more neutrons than U- 235. Atomic mass is the sum total of the nucleons or protons and neutrons.
What are Isotopes?
They are the different variants of the same molecules which have the same number of protons but the different number of neutrons.
Atomic mass is the sum total of the nucleons or protons and neutrons. The atomic number of Uranium is 92, the rest of the mass comes from neutrons.
Therefore, the U- 238 has more neutrons than U- 235.
Learn more about Isotopes:
https://brainly.com/question/9099776
In the compound Na3PO4, which two atoms are involved in the formation of a covalent bond?
Answers
Answer:
P-O.
Explanation:
Hello there!
In this case, according to the definition of the covalent bond, which involves valence electrons sharing, occurring specially between nonmetals, we infer that the P-O bond is the most likely to be covalent, because the P-Na bond is not present and the O-Na bond is ionic.
Regards!
The mass number of an atom can be calculated from the
Answers
Mass number (A) =protons (p+) + neutrons(n°)