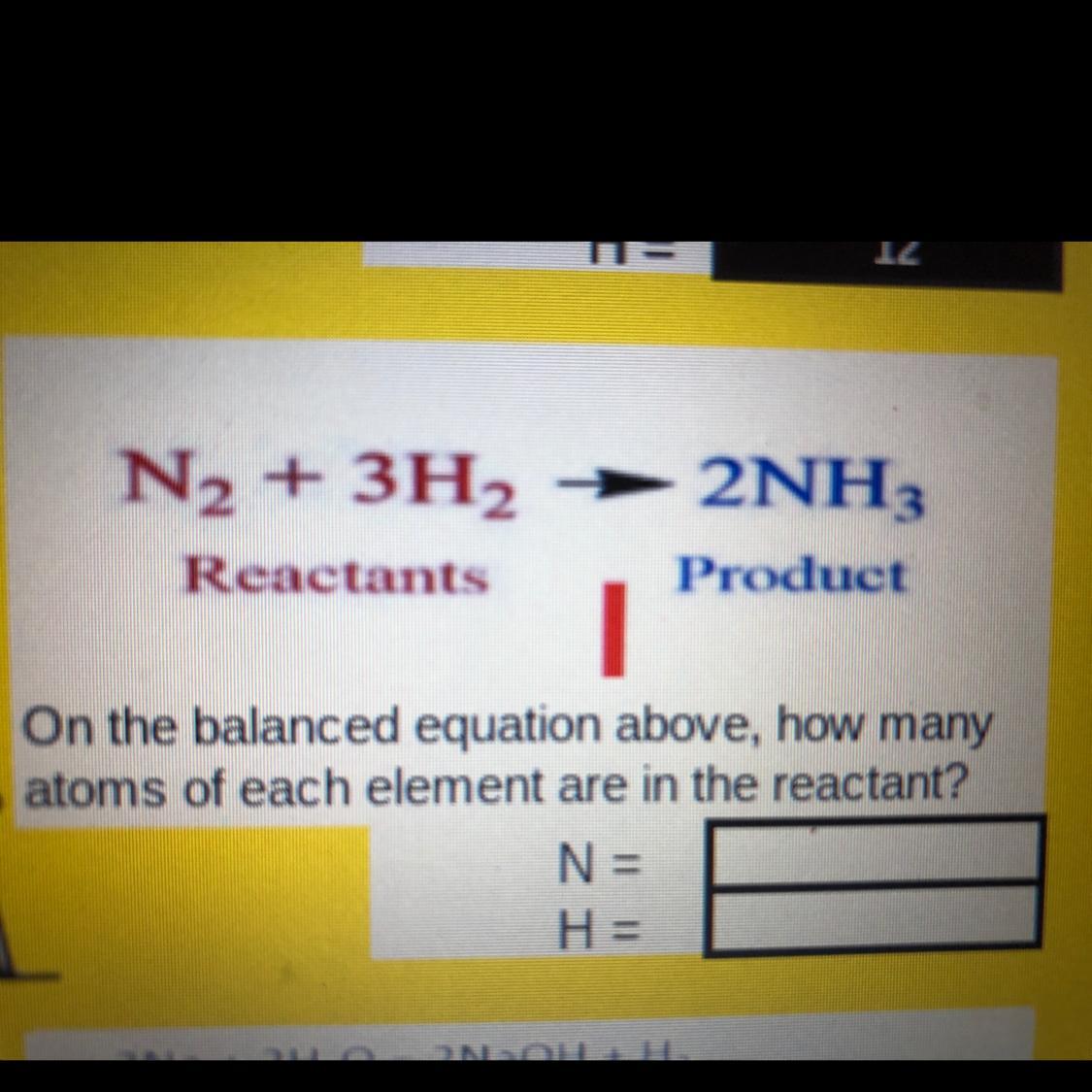
Answers
Answer:
N=2
H=6
Explanation:
1.Balance a chemical equation in terms of moles.
2.Use the balanced equation to construct conversion factors in terms of moles.
3.Calculate moles of one substance from moles of another substance using a balanced chemical equation.
The law of conservation of matter says that matter cannot be created or destroyed. In chemical equations, the number of atoms of each element in the reactants must be the same as the number of atoms of each element in the products.
(P.s it could also be where you have to solve it in which you have to simplify it first then solve it.) like adding them all up.
Hope this is the answer. :)
Related Questions
________ and ________ fibres are obtained from animals
Answers
Answer:
Explanation:
Silk and wool
Answer:
silk and wool fibers are obtained from animals
Explanation:
good luck :)
Hereditary information is passed on from:
O nucleus to nucleus
O cell to cell
O cell to nucleus
O nucleus to cell
Answers
Answer:
D - Nucleus to Cell
Explanation:
Hope it helps!!
:D
If the density of aluminum is 2.7g/ml, what is the volume of 12.0g?
Answers
Answer:
The answer is 4.44 mLExplanation:
The volume of a substance when given the density and mass can be found by using the formula
[tex]volume = \frac{mass}{density} \\ [/tex]
From the question we have
[tex]volume = \frac{12}{2.7} \\ = 4.4444444....[/tex]
We have the final answer as
4.44 mLHope this helps you
A liquid has a volume of 62.7 mL and a density of 2.59 g/mL. What is its mass? (show all work)
Answers
Answer:
162.4
Explanation:
The formula for mass is density* volume so 62.7 multiplied by 2.59 equals 162.393. then you round so your anwser would be 162.4
using this chemical might discolour equipment and surfaces
Answers
Answer: do not know how to answer this
Explanation:
Level 1 01 Which correctly pairs the outside particles with their charge? A. Electrons: Positive B. Protons: Positive C. Neutrons: Neutral D. Electrons: Negative
Answers
Answer:
D. Electrons: Negative.
Explanation:
Hello, happy to help you today!
In this case, by considering the Bohr's atomic model in which atom is composed by a nuclei containing both protons and neutrons which are positively and neutrally charged respectively and surrounding electrons assembled in orbits or levels of energy which are negatively charged in order to provide a balance to the atom, the correct statement is: D. Electrons: Negative. Also consider the Bohr's model on the attached picture.
My best regards to you!
What is another term for the limbic system?:
A. primitive brain
B. logical brain
C. gender center
D. memory center
Answers
Answer:
a
Explanation:
because the sytem it si locate at the bothe side
Which statement best demonstrates how data from a global positioning system (GPS) can be used to lessen the effects of a
wildfire? (1 point)
GPS data can be used by people to quickly evacuate an area because of a wildfire
GPS data can be used by scientists to predict weather patterns that can lead to a wildfire
GPS data can be used by firefighters to identify the boundaries of a wildfire
GPS data can be used by first responders to calculate the safest route to a wildfire
Answers
Answer: here is your answer
Explanation: You are visiting your Grandmother and notice that she is eating a balanced diet, taking vitamins, getting the proper amount of sleep and is not overweight. Despite her healthy lifestyle, she appears run down and tired. You realize that it's due to her lack of physical activity. Write a convincing letter to your grandma explaining the benefits of participating in regular physical activity.
__________ have the lowest ionization energies of the groups listed
PLZ HELP I'LL AWARD BRAINLIEST
Answers
Answer: have the lowest ionization energies of the groups listed
PLZ HELP I'LL AWARD BRAINLIEST
Explanation:
I don't know what category to put this question in, but I attached a photo of it. Can someone please help me answer it?
Answers
Analysing the question:
To calculate the density of a material, we need its mass and volume
We are given:
Mass of sample = 21 grams
dimensions of the sample = 1 * 1 * 2 = 2 cm³
Calculating the density:
Density = Mass of sample / volume of sample
Replacing the variables
Density = 21 / 2
Density = 10.5 g / cm³
Determining the Material:
From the table provided, we can see that the density of Silver is 10.5 g/cm³
Therefore, the material is Silver
____ KClO3 = ____ KCl + ____ O2
Answers
Answer:
2KCIO3=2KCI+3O2
Explanation:
1. equalize right side O with left side O- Right side we have 3 and left only 2 so we add 3 in front(on left side)(3*2=6 O on both sides)
2.we add 2 on right side so now we have 6 O on left and 6 O on right and 2KCI
3.Add 2 on right side KCI so on both sides we got 2 KCI
4. This is low wet land.
A. swamp
B. island
C. desert
D. peninsula
5. Japan is one of these.
A. island
B. peninsula
C. swamp
D. desert
Answers
Answer:
swap
Explanation: because it is a low wet land
Answer:
4. swamp
5. Island
Plz mark brainliest:)
State Hess' law of constant heat summation.
(b) Calculate the enthalpy of formation of CH4 from the following data:
i) C(s) + O2(g) → CO2(g); ∆H = -393.7 kJ/mol
ii) H2(g) + 1⁄2 O2(g) → H2O(l); ∆H = -285.8 kJ/mol
iii) CH4(g) + 2 O2(g)→ CO2(g) + 2H2O(l); ∆H = -890.4 kJ/mol
Answers
Answer:
-74.6 kj/mol
Explanation:
you can see the answer at the pic
Calculate the following quantity: molarity of a solution prepared by diluting 49.16 mL of 0.0270 M ammonium sulfate to 525.00 mL.
Answers
Answer:
2.528x10⁻³M
Explanation:
Molarity is an unit of concentration used in chemistry. Is defined as the moles of solute per liter of solution.
To find the molarity of the solution we need to determine the moles of ammonium sulfate present in the initial 49.16mL solution and, with total volume, we can find the molarity, thus:
Moles ammonium sulfate:
49.16mL = 0.04916L * (0.0270 moles / L) =
1.327x10⁻³moles ammonium sulfate
These moles are present in 525.0mL = 0.525L. Thus, molarity of the solution will be:
1.327x10⁻³moles ammonium sulfate / 0.525L =
2.528x10⁻³MExpress the following numbers in scientific notation:
872
Answers
Answer:
The number 872 would be written as,
8.72× 10²
Explanation:
Scientific notation is used to express the large value in short form.
The number in scientific notation have two parts.
First part:
The digits (decimal point will place after first digit)
Second part:
× 10 ( the power which put the decimal point where it should be)
For example the number 872 would be written as
8.72× 10²
Another example:
6324.4 in scientific notation will be written as = 6.3244 × 10³
To vaporize/condense a substance, does the substance have to absorb or release heat?
Answers
Answer: it would release heat because the thermal energy it absorbed to become a gas. so it would release heat. hope this helps :)
Explanation:
i’m so lost please help
Answers
C=4
D=-19
Explanation:
2exp-25/5exp-7= 4exp-19
8. Why is the magnetic force considered to be a noncontact force?
Answers
Answer:
Magnetic forces are non contact forces; they pull or push on objects without touching them. Magnets are only attracted to a few 'magnetic' metals and not all matter. Magnets are attracted to and repel other magnets.
Hope this helps!!!
Explanation:
plsss help!!!!! I'll give u brainlest and 10 points
Answers
Answer:
I would say it is true
Explanation:
The reaction between HCl and KOH results in an increase in temperature in the solution. Select the correct statement from the list below.
a) this is an endothermic reaction
b) this is a phase change reaction
c) this is a vaporization reaction
d) this is an exothermic reaction
Answers
Answer:
d) this is an exothermic reaction.
Explanation:
The reaction between HCl and KOH results in an increase in temperature in the solution. Select the correct statement from the list below.
a) this is an endothermic reaction . NO. This would cause a decrease in the temperature of the solution.
b) this is a phase change reaction . NO. All the species remain in the aqueous phase.
c) this is a vaporization reaction . NO. All the species remain in the aqueous phase.
d) this is an exothermic reaction. YES. The reaction releases heat, so it is exothermic.
‘ASAP’What do the different categories of hurricanes represent?
O Air density
O Humidity
O Pressure
O Wind speeds
Answers
Answer:
Sorry I do not know the answer but I do know its not c (pressure)
Explanation:
I took a quiz with that question and I got it wrong my other guess is air density though
Answer:
Wind Speeds
Explanation:
im awnsering late cuz i need pointsss
magnesium: atomic number
Answers
Answer:
Magnesiums atomic number is 12
please give me brainliest!
God bless!
"Calculate the pH of an aqueous solution at 25°C that is (a) 0.15 M in HCl. 0.82 (b) 3.7 M in HNO3. -0.57 (c) 6.9 × 10−4 M in HClO4."
Answers
Answer:
See explanation
Explanation:
The pH of a solution is defined as the negative logarithm of hydrogen ion concentration.
For HCl
pH= - log[0.15] = 0.82
For HNO3
pH= -log[3.7] = -0.57
For HClO4
pH= - log [6.9 × 10^-4] = 3.16
Use the periodic table to identify the chemical symbol or name for each element below.
zirconium:
zr
rhenium:
re
: As
: K
tin:
yttrium:
: Yb
Answers
Answer:
zirconium is Zr
rhenium is Re
As is arsenic
K is potassium
tin is Sn
yttrium is Y
ytterbium is Yb
Explanation:
With the help of the periodic table, the identification of the chemical symbol or name for each element is represented as follows:
Zirconium: Zr. Rhenium: Re. Arsenic: As.Potassium: K.Tin: Sn. Yttrium: Y. Ytterbium: Yb. What are the chemical elements?Chemical elements may be defined as any type of substance that cannot be further decomposed into simpler substances through the utilization of other ordinary chemical processes or external factors. These elements possess specific physical as well as chemical properties distinctly.
In the modern periodic table, each element is arranged on the basis of its increasing atomic number. Each element possesses its unique symbol, atomic mass, and physical properties like boiling points, melting points, density, etc.
Therefore, with the help of the periodic table, the identification of the chemical symbol or name for each element is well represented above.
To learn more about the Periodic table, refer to the link:
https://brainly.com/question/15987580
#SPJ2
What is the volume of 11.2 g of O2 at 7.78 atm and 415 K?
Answers
Answer:
1.53 L
Explanation:
Step 1: Given data
Mass of oxygen (m): 11.2 gPressure (P): 7.78 atmTemperature (T): 415 KIdeal gas constant (R): 0.0821 atm.L/mol.KStep 2: Calculate the moles (n) corresponding to 11.2 g of oxygen
The molar mass of oxygen is 32.00 g/mol.
11.2 g × (1 mol/32.00 g) = 0.350 mol
Step 3: Calculate the volume of oxygen
We will use the ideal gas equation.
P × V = n × R × T
V = n × R × T / P
V = 0.350 mol × (0.0821 atm.L/mol.K) × 415 K / 7.78 atm
V = 1.53 L
The initial pressure of a mixture of C6H6 and an excess of H2 in a rigid vessel is 1.21 atm. A catalyst is introduced. After the reaction reaches completion, the temperature is restored to its initial value. The final pressure in the vessel is 0.839 atm. What was the mole fraction of C6H6 in the original mixture
Answers
Answer:
mole fraction of C6H6 = 0.613 atm
Explanation:
The equation for this reaction is :
[tex]C_6H_6 _{(g)} + 3H_2_{(g)} \to C_6H_{12}_{(g)}[/tex]
Initial P₁ P₂ 0
Final 0 P₂ -P₁/2 P₁
After completion of the reaction;
P₁ + P₂ = 1.21 atm ----- (1)
P₂ - P₁/2 + P₁ = 0.839 atm
P₂ + P₁/2 = 0.839 atm ----- (2)
Subtracting (2) from (1); we have:
P₁/2 = 0.371
P₁ = 0.742 atm
From(1)
P₁ + P₂ = 1.21 atm
0.742 atm + P₂ = 1.21 atm
P₂ = 1.21 atm - 0.742 atm
P₂ = 0.468 atm
Thus, the partial pressure of C6H6 = 0.742 atm
∴
Partial pressure = Total pressure × mole fraction of C6H6
mole fraction of C6H6 = Partial pressure / Total pressure
mole fraction of C6H6 = 0.742 atm / 1.21 atm
mole fraction of C6H6 = 0.613 atm
A student asks why the ashes from a fire have a much lower mass than the wood that was burned.
Which is the correct answer to the student’s question?
Gases are released into the air.
Atoms in the wood are destroyed.
Heat causes the molecules to change color.
Water inside the wood solidifies.
(I am not in college)
Answers
What can you infer about the likely occurrence of the other isotopes in each of the
above elements? Explain your reasoning.
Answers
Answer:
Most common has the same or closest mass because it is closer to the original’s mass.
Explanation:
I got it from the answer key
Read more here:
https://brainly.com/question/1598931
Which element is classified as a noble gas?
Answers
Answer:
The elements with completely filled shells are classified as noble gases
That is why we only see noble gases on the rightmost corner of the periodic table, it is because they have the maximum number of electrons in a shell
Examples of noble gases
Helium , Neon , Argon and Krypton are some examples of noble gases
Answer:
D. (Xe) XenonExplanation:
I JUST TOOK THE TEST!
what does celery, a wooden spoon, and oil/gasoline have in common?
Answers
Answer:
All of them are organic compounds which have carbon as their main atom in the structure.
Explanation:
Hello.
In this case, since organic chemistry is the study of all the compounds having the carbon atom as their main atom, all the vegetables, animals, an in general, living things are composed by lipids, proteins, and other organic substances with this feature. Moreover, wood-based materials are mainly composed by lignin which is an organic polymer also having carbon as the main atom. In addition, oil and gasoline are organic chemical compounds with a lot of applications in daily life which also contain carbon atoms in their structure.
In such a way, a celery, a wooden spoon, and oil/gasoline have the carbon atom in common as their main atom in their chemical structures.
Best regards.